UW-Madison scientists guide human skin cells to embryonic state
In a paper to be published Nov. 22 in the online edition of the journal Science, a team of University of Wisconsin–Madison researchers reports the genetic reprogramming of human skin cells to create cells indistinguishable from embryonic stem cells.
“The induced cells do all the things embryonic stem cells do. It’s going to completely change the field.”
James Thomson, professor of anatomy and the scientist who first coaxed stem cells from human embryos in 1998
The finding is not only a critical scientific accomplishment, but potentially remakes the tumultuous political and ethical landscape of stem cell biology as human embryos may no longer be needed to obtain the blank slate stem cells capable of becoming any of the 220 types of cells in the human body. Perfected, the new technique would bring stem cells within easy reach of many more scientists as they could be easily made in labs of moderate sophistication, and without the ethical and legal constraints that now hamper their use by scientists.
The new study was conducted in the laboratory of UW–Madison biologist James Thomson, the scientist who first coaxed stem cells from human embryos in 1998. It was led by Junying Yu of the Genome Center of Wisconsin and the Wisconsin National Primate Research Center.
Related audio
Listen to James Thomson’s opening statement at the Nov. 21 press briefing.
Related story
Reprogramming the debate: stem-cell finding alters ethical controversy
When UW–Madison researchers succeeded in reprogramming skin cells to behave like embryonic stem cells, they also began to redefine the political and ethical dynamics of the stem-cell debate, a leading bioethicist says.
Read more
“The induced cells do all the things embryonic stem cells do,” explains Thomson, a professor of anatomy in the University of Wisconsin School of Medicine and Public Health. “It’s going to completely change the field.”
In addition to exorcising the ethical and political dimensions of the stem cell debate, the advantage of using reprogrammed skin cells is that any cells developed for therapeutic purposes can be customized to the patient.
“They are probably more clinically relevant than embryonic stem cells,” Thomson explains. “Immune rejection should not be a problem using these cells.”
An important caveat, Thomson notes, is that more study of the newly-made cells is required to ensure that the “cells do not differ from embryonic stem cells in a clinically significant or unexpected way, so it is hardly time to discontinue embryonic stem cell research.”
The successful isolation and culturing of human embryonic stem cells in 1998 sparked a huge amount of scientific and public interest, as stem cells are capable of becoming any of the cells or tissues that make up the human body.
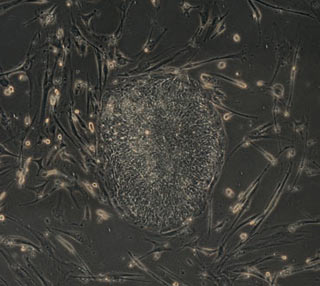
The scientific team from the University of Wisconsin–Madison created genetic modifications in skin cells to induce the cells into what scientists call a pluripotent state — a condition that is essentially the same as that of embryonic stem cells. Junying Yu, James Thomson and their colleagues introduced a set of four genes into human fibroblasts, skin cells that are easy to obtain and grow in culture.
High-resultion images related to this story
The potential for transplant medicine was immediately recognized, as was their promise as a window to the earliest stages of human development, and for novel drug discovery schemes. The capacity to generate cells that could be used to treat diseases such as Parkinson’s, diabetes and spinal cord injuries, among others, garnered much interest by patients and patient advocacy groups.
But embryonic stem cells also sparked significant controversy as embryos were destroyed in the process of obtaining them, and they became a potent national political issue beginning with the 2000 presidential campaign. Since 2001, a national policy has permitted only limited use of some embryonic stem cell lines by scientists receiving public funding.
In the new study, to induce the skin cells to what scientists call a pluripotent state, a condition that is essentially the same as that of embryonic stem cells, Yu, Thomson and their colleagues introduced a set of four genes into human fibroblasts, skin cells that are easy to obtain and grow in culture.
Finding a combination of genes capable of transforming differentiated skin cells to undifferentiated stem cells helps resolve a critical question posed by Dolly, the famous sheep cloned in 1996. Dolly was the result of the nucleus of an adult cell transferred to an oocyte, an unfertilized egg. An unknown combination of factors in the egg caused the adult cell nucleus to be reprogrammed and, when implanted in a surrogate mother, develop into a fully formed animal.
The new study by Yu and Thomson reveal some of those genetic factors. The ability to reprogram human cells through well defined factors would permit the generation of patient-specific stem cell lines without use of the cloning techniques employed by the creators of Dolly.
“These are embryonic stem cell-specific genes which we identified through a combinatorial screen,” Thomson says. “Getting rid of the oocyte means that any lab with standard molecular biology can do reprogramming without difficulty to obtain oocytes.”
Although Thomson is encouraged that the new cells will speed new cell-based therapies to treat disease, more work is required, he says, to refine the techniques through which the cells were generated to prevent the incorporation of the introduced genes into the genome of the cells. In addition, to ensure their safety for therapy, methods to remove the vectors, the viruses used to ferry the genes into the skin cells, need to be developed.
Using the new reprogramming techniques, the Wisconsin group has developed eight new stem cell lines. As of the writing of the new Science paper, which will appear in the Dec. 21, 2007 print edition of the journal Science, some of the new cell lines have been growing continuously in culture for as long as 22 weeks.
The new work was funded by grants from the Charlotte Geyer Foundation and the National Institutes of Health. In addition to Yu and Thomson, authors of the new study include Maxim A. Vodyanik, Kim Smuga-Otto, Jessica Antosiewicz-Bourget, Jennifer L. Frane and Igor I. Slukvin, all of UW–Madison; and Shulan Tian, Jeff Nie, Gudrun A. Jonsdottir, Victor Ruotti and Ron Stewart, all of the WiCell Research Institute.
More information: Learn more about stem cell and regenerative medicine research at UW–Madison by visiting the Stem Cell and Regenerative Medicine Center Web site.
Tags: audio, biosciences, business, genetics, health & medicine, international, research, stem cells




