Waistline growth on high-carb diets linked to liver gene
Experts have been warning for years that foods loaded with high-fructose corn syrup and other processed carbohydrates are making us fatter. Now, a UW–Madison study has uncovered the genetic basis for why this is so.
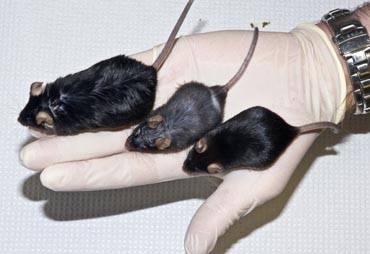
Research led by biochemistry and nutritional sciences professor James Ntambi shows that mice, at right, lacking a gene called SCD-1 in their livers stay skinny on a high-carbohydrate diet, but grow plump, like the mouse at left, on a high-fat one. The findings suggest that the gene’s action in the liver is what causes weight gain on diets rich in carbohydrates, because deleting the gene protects the mice from getting fat when fed starchy and sugary foods.
Photo: courtesy Biochemistry Media Center
Writing in the December issue of Cell Metabolism, a team led by biochemistry and nutritional sciences professor James Ntambi reports that a gene in the liver, called SCD-1, is what causes mice to gain weight on a diet laden with carbohydrates. The gene encodes the enzyme SCD, whose job is to synthesize fatty acids that are a major component of fat.
When the scientists fed a starch- and sugar-rich diet to mice lacking SCD-1 in the liver, the extra carbohydrates were broken down rather than being converted into fat and stored — keeping the mice skinny. Meanwhile, control mice with normal gene activity grew plump on the same food.
"It looks like the SCD gene in the liver is responsible for causing weight gain in response to a high-carbohydrate diet, because when we take away the gene’s activity the animals no longer gain the weight," says Ntambi. "These findings are telling us that the liver is a key tissue in mediating weight gain induced by excess carbohydrates."
The results could have implications for stemming the skyrocketing obesity problem in people, Ntambi adds. He explains that people pack on pounds in two ways, one of which is to eat extra fat, which then accumulates in adipose, or fat, tissue. But the main cause of weight gain is excess carbohydrates, because they trigger the body to produce new fat.
Blocking SCD’s action in the liver could therefore offer another means to help people lose weight, Ntambi says, especially since obese people appear to have higher levels of the enzyme than do thin people.
"We think that obese individuals, in general, may have higher SCD activity in both the liver and in adipose tissue," he says. "So they may have a higher capability of converting carbohydrate into fat."
High-carbohydrate diets have become exceedingly common not only in western nations but also in the developing world, as sugary ingredients such as high-fructose corn syrup have crept into all sorts of processed foods, including soft drinks, baked goods, condiments — even supposedly healthy items such as low-fat, fruit yogurt. What Ntambi’s team has now demonstrated is how those diets can act directly on a gene to boost fat synthesis and storage.
"This is a very good example of a diet-gene interaction," he says.
The current study builds on previous work, in which Ntambi and his colleagues created mice that lacked SCD-1 everywhere in the body, including the liver, muscle, brain, pancreas and adipose tissue. No matter how much they ate, the mice didn’t gain weight on either a high-fat or a high-carbohydrate diet. "But it was very difficult to tell which tissue was responsible for the effect," says Ntambi.
To tease this out, he and his colleagues subsequently bred mice that lacked SCD-1 in the liver only and placed them on either a high-fat diet or a high-carbohydrate, low-fat one. Much to their surprise, the mice on the high-fat diet gained weight just as quickly as normal, control mice.
"This suggests that in weight gain induced by a high-fat diet, other tissues beyond the liver are involved," says Ntambi.
In contrast, the mice stayed thin when they feasted on food heavy in starch and table sugar, or sucrose. They were also protected from the condition known as fatty liver. Ntambi thinks what’s happening is that in the absence of SCD, the liver has no way to convert surplus carbohydrates into fat, causing the body to break them down instead.
The findings also highlight the central role of the enzyme and its main product, a fatty acid known as oleic acid, in overall carbohydrate metabolism, he adds. For example, mice lacking SCD could no longer make glucose — the sugar burned by cells for energy — leading to abnormally low blood sugar levels, or hypoglycemia. They also weren’t able to make glycogen, a short-term storage form of glucose.
"It looks to us that if you don’t have enough oleic acid — which the SCD enzyme makes — then the carbohydrate does not proceed through normal glucose metabolism," says Ntambi. As further evidence of this, when the scientists supplemented the mouse diets with oleic acid, normal metabolism was restored.
In both mice and people, on the other hand, eating lots of carbohydrate appears to boost SCD activity, leading to a glut of oleic acid, increased fat storage — and, over time, obesity.
"Too much carbohydrate is not good," says Ntambi. "That’s basically what we are saying."
Ntambi’s study was supported by the National Institutes of Health and the American Heart Association.




