Sea urchin yields a key secret of biomineralization
The teeth and bones of mammals, the protective shells of mollusks, and the needle-sharp spines of sea urchins and other marine creatures are made-from-scratch wonders of nature.
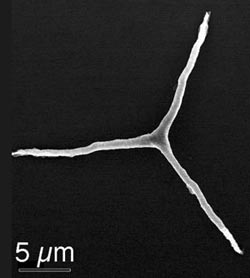
Looking like the hood ornament of a Mercedes-Benz, a single calcite crystal made by a sea urchin larva, sets the stage for the development of the marine animal’s flint-hard spicule, the spiny structure that serve’s as the animal’s endoskeleton. Using the soft X-rays produced by synchrotron radiation, Wisconsin scientists observed how the sea urchin transformed amorphous calcium carbonate into calcite, the biomineral that is the stuff of sea shells.
Photo: courtesy Pupa Gilbert
Used to crush food, for structural support and for defense, the materials of which shells, teeth and bones are composed are the strongest and most durable in the animal world, and scientists and engineers have long sought to mimic them.
Now, harnessing the process of biomineralization may be closer to reality as an international team of scientists has detailed a key and previously hidden mechanism to transform amorphous calcium carbonate into calcite, the stuff of seashells. The new insight promises to inform the development of new, superhard materials, microelectronics and micromechanical devices.
In an Oct. 27 report in the Proceedings of the National Academy of Sciences (PNAS), a group led by UW–Madison physicist Pupa Gilbert describes how the lowly sea urchin transforms calcium carbonate — the same material that forms “lime” deposits in pipes and boilers — into the crystals that make up the flint-hard shells and spines of marine animals. The mechanism, the authors write, could “well represent a common strategy in biomineralization….”
“If we can harness these mechanisms, it will be fantastically important for technology,” argues Gilbert, a UW–Madison professor of physics. “This is nature’s bottom-up nanofabrication. Maybe one day we will be able to use it to build microelectronic or micromechanical devices.”
Gilbert, who worked with colleagues from Israel’s Weizmann Institute of Science, the University of California at Berkeley and the Lawrence Berkeley National Laboratory, used a novel microscope that employs the soft-X-rays produced by synchrotron radiation to observe how the sea urchin builds its spicules, the sharp crystalline “bones” that constitute the animal’s endoskeleton at the larval stage.
Similar to teeth and bones, the sea urchin spicule is a biomineral, a composite of organic material and mineral components that the animal synthesizes from scratch, using the most readily available elements in sea water: calcium, oxygen and carbon. The fully formed spicule is composed of a single crystal with an unusual morphology. It has no facets and within 48 hours of fertilization assumes a shape that looks very much like the Mercedes-Benz logo.
These crystal shapes, as those of tooth enamel, eggshells or snails, are very different from the familiar faceted crystals grown through non-biological processes in nature. “To achieve such unusual — and presumably more functional — morphologies, the organisms deposit a disordered amorphous mineral phase first, and then let it slowly transform into a crystal, in which the atoms are neatly aligned into a lattice with a specific and regular orientation, while maintaining the unusual morphology,” Gilbert notes.
The question the Wisconsin physicist and her colleagues sought to answer was how this amorphous-to-crystalline transition occurs. The sea urchin larval spicule is a model system for biominerals, and the first one in which the amorphous calcium carbonate precursor was discovered in 1997 by the same Israeli group co-authoring the current PNAS paper. A similar amorphous-to-crystalline transition has since been observed in adult sea urchin spines, in mollusk shells, in zebra fish bones and in tooth enamel. The resulting biominerals are extraordinarily hard and fracture resistant, compared to the minerals of which they are made.
“The amorphous minerals are deposited and they are completely disordered,” Gilbert explains. “So the question we addressed is ‘how does crystallinity propagate through the amorphous mineral?'”
To answer it, Gilbert and her colleagues observed spicule development in 2- to 3-day-old sea urchin larvae. The sea urchin spicule is formed inside a clump of specialized cells and begins as the animal lays down a single crystal of calcite in the form of a rhombohedral seed, from which the rest of the spicule is formed. Starting from the crystalline center, three arms extend at 120 degrees from each other, as in the hood ornament of a Mercedes-Benz. The three radii are initially amorphous calcium carbonate, but slowly convert to calcite.
“We tried to find evidence of a massive crystal growth, with a well defined growth front, propagating from the central crystal through the amorphous material, but we never observed anything like that,” Gilbert says. “What we found, instead, is that 40-100 nanometer amorphous calcium carbonate particles aggregate into the final morphology. One starts converting to crystalline calcite, then another immediately adjacent converts as well, and another, and so on in a three-dimensional domino effect. The pattern of crystallinity, however, is far from straight. It resembles a random walk, or a fractal, like lightning in the sky or water percolating through a porous medium,” explains Gilbert.
The new work, according to Gilbert, brings science a key step closer to a thorough understanding of how biominerals form and transform. Knowing the step-by-step process may permit researchers to develop new crystal structures that can be used in applications ranging from new microelectronic devices to medical applications.
The new study was funded by the National Science Foundation and the U.S. Department of Energy.
Tags: biosciences, physics, research




