Rare disease yields clues about broader brain pathology
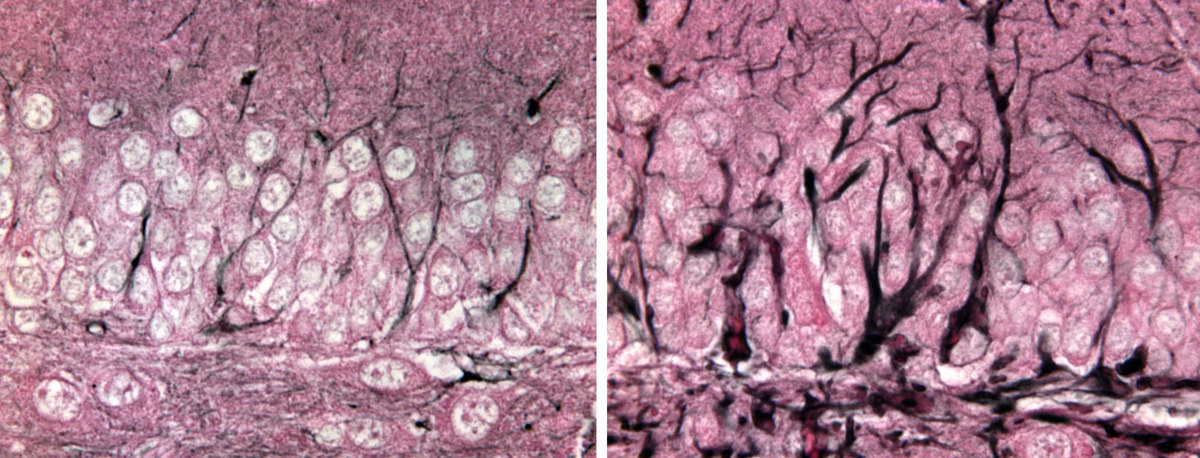
A mutant gene that causes the deadly Alexander disease creates an overgrowth of the protein GFAP in mouse brain cells called astrocytes (right) compared to normal brain cells (left).
Alexander disease is a devastating brain disease that almost nobody has heard of — unless someone in the family is afflicted with it. Alexander disease strikes young or old, and in children destroys white matter in the front of the brain. Many patients, especially those with early onset, have significant intellectual disabilities.
Regardless of the age when it begins, Alexander disease is always fatal. It typically results from mutations in a gene known as GFAP (glial fibrillary acidic protein), leading to the formation of fibrous clumps of protein inside brain cells called astrocytes.
Classically, astrocytes and other glial cells were considered “helpers” that nourish and protect the neurons that do the actual communication. But in recent years, it’s become clear that glial cells are much more than passive bystanders, and may be active culprits in many neurological diseases.
Now, in a report in the Journal of Neuroscience, researchers at UW–Madison show that Alexander disease also affects neurons, and in a way that impacts several measures of learning and memory.
“Think of a garden where your green beans never sprouted. Was it too cold for them to sprout, or was there another problem? Something similar is happening with these neural stem cells.”
Tracy Hagemann
Mice were engineered to contain the same mutation in GFAP that is found in human patients. Their astrocytes spontaneously increased production of GFAP, the same response found after many types of injury or disease in the brain. In Alexander disease, the result is an increase in mutant GFAP that is “toxic to the cell, and unfortunately astrocytes respond by making more GFAP,” says first author Tracy Hagemann, an associate scientist with the university’s Waisman Center.
While GFAP is usually found in astrocytes, it also occurs in neural stem cells, a population of cells that persist in some areas of the brain to continually spawn new neurons throughout adulthood. In the mouse versions of Alexander disease, neural stem cells are present, but they fail to develop into neurons, Hagemann says. “Think of a garden where your green beans never sprouted. Was it too cold for them to sprout, or was there another problem? Something similar is happening with these neural stem cells. They are present, but inert, and we’re not sure why.”
The shortage of new neurons could explain why the mice with excess GFAP failed a test that required them to remember the location of a submerged platform in a tub of water.
The report is “the first to suggest that the problems in Alexander disease extend beyond just the white matter and astrocytes, and may provide a clue to the problems with learning and memory that are such prominent features in the human disease,” says lab leader Albee Messing, a professor of comparative biosciences in the UW School of Veterinary Medicine.
Eventually, Hagemann says, the work may illuminate the role of astrocyte dysfunction in ALS, Parkinson’s and Alzheimer’s disease.
One immediate question that the team will try to answer is whether the same defect in stem cells can be found in autopsy samples stored over many years to allow just this kind of investigation.
Still to be clarified is whether the mutation affects the neural stem cells directly, or whether it acts through other astrocytes that are nearby. “We do know that the astrocytes become activated with this GFAP mutation,” Hagemann says. “That activation — a kind of inflammation — could be making the environment hostile to young neurons. Or the mutation could be changing the neural stem cells themselves in some other way.
“Medicine advances by teasing things apart,” says Hagemann. “A single mutation can work in different ways — through different chains of cause and effect leading to different symptoms of a disease. In this case it’s like the old question of nature versus nurture. Was the stem cell born bad — was it genetically doomed? Or were the reactive astrocytes in the neighborhood a toxic influence? Or both? This is an important question for Alexander disease and other brain deteriorating disorders, especially with the current focus on stem cells as a source for new neurons and therapy.”
Already, the Waisman group is screening drugs that might slow GFAP production. Eventually, Hagemann says, the work may illuminate the role of astrocyte dysfunction in other neural diseases featuring aggregates of misformed proteins, including ALS, Parkinson’s, and Alzheimer’s disease.




