Five questions with Su-Chun Zhang, forger of brain cells
 Su-Chun Zhang, a Waisman Center researcher and UW School of Medicine and Public Health professor of neurology and neuroscience, was the first in the world to craft human brain cells from human embryonic stem (ES) cells, and later from the related induced pluripotent (iPS) cells. In light of the 20th anniversary of James Thomson’s derivation of human ES cells, we had some questions for a founder of stem cell neuroscience.
Su-Chun Zhang, a Waisman Center researcher and UW School of Medicine and Public Health professor of neurology and neuroscience, was the first in the world to craft human brain cells from human embryonic stem (ES) cells, and later from the related induced pluripotent (iPS) cells. In light of the 20th anniversary of James Thomson’s derivation of human ES cells, we had some questions for a founder of stem cell neuroscience.
What got you into the stem cell business in the first place?
My work was always research, but I was trying to find a way to deal with patient problems; I came from medical school. With brain cells, the source of material is critical. You could study canine or rodent neural stem cells, but they are not human. For neural cells, you have to take them from the brain, which means from abortions or from surgical tissue. I was looking to overcome these ethical and practical issues, and I started working with Jamie [Thomson] even before his human ES cell discovery.

“The pure science is fascinating, but when I talk to patients, I see their anxiety, their desire to move ahead,” says Su-Chun Zhang. “But as a scientist, or as a doctor, the number one thing is to make sure you do not create harm, and so we move cautiously.” UW Health / John Maniaci
After I got the ES cells and watched them differentiate, I quickly saw the neural cells. The neural stem cells line up beautifully in a neural tube-like rosette. The neural tube is the structure from which our brain and spinal cord form. I grabbed Jamie to have a look, and he told me he’d seen those cells many times. I came from the neural field, so I immediately knew what I was seeing. But Jamie was an expert in stem cells, so he did not pay attention. It was remarkable, very fascinating to me, rewarding. In science this happens all the time. Science requires people from different fields to generate something new.
It’s been 20 years now and stem cells have not yet provided any treatments for human neurological problems. Why is it so hard to derive such treatments?
When we started, we thought these cells could be extremely useful for patients, but the problem is extremely difficult. We spent many years arguing about the morality and legality of using embryonic stem cells. That’s over, but the brain is a very special organ where the cells are wired very precisely, and that complicates the work greatly. The first step is getting a pure population of cells of the exact type of neuron.
Parkinson’s, for example, results from the death of neurons deep in the brain that make a neurotransmitter called dopamine. So to treat Parkinson’s, you need the midbrain type of dopamine-creating neuron, not just any kind of dopamine neuron. Although we can’t yet produce pure populations of those cells, we’re getting close.
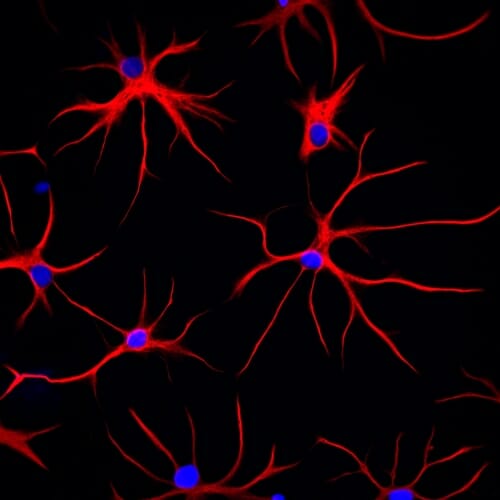
These astrocytes were derived from human stem cells. Red shows the structural protein, GFAP, which gives the cell its distinctive starlike shape. DNA in the cell nucleus appears blue. Jeffrey Jones / Zhang lab
The overriding issue is safety. It’s not enough to prove that transplanted cells don’t generate a tumor; we also need to ensure they don’t create other problems. The brain is almost unfathomably complex, and if transplanted cells wire incorrectly, that can be worse than useless. Animals seem easier compared to humans. We can get cell replacement to treat the animal model of Parkinson’s, but there we only look at motor function. With a human being, we need to consider thinking, reasoning, memory, talking, emotions, to name a few.
Are you frustrated?
Yes, because I always want to move it to the clinic. The pure science is fascinating, but when I talk to patients, I see their anxiety, their desire to move ahead. But as a scientist, or as a doctor, the number one thing is to make sure you do not create harm, and so we move cautiously. Look around: For any new drug development, 15 years is a common time course. Yet here we are dealing with living cells, which are vastly more complex. And once you put them into the brain, you cannot scoop them out if something goes wrong.
What have stem cells taught us about how human brains develop and work?
It turns out that human brain cells behave very differently than animal cells. For example, we published a paper saying that the genes that control brain development are different in humans compared to animals. This may explain why, most of the time, drugs that work in animals do not help patients, particularly for neural problems.

Kaiping Xu dispenses cells at BrainXell, a UW–Madison spinoff founded to pursue Su-Chun Zhang’s processes for developing stem cells into neural cells. Photo: David Tenenbaum
Take the fatal disease ALS, which is caused by a degeneration of motor neurons. There are a dozen effective treatments for animal models, but none of the clinical trials have been effective for patients. This pains me, but it also motivates me.
With the development of iPS cells, you can get stem cells from patients and look at the disease process. Previously, we’ve had to use animal models, transgenic cells or cells with genes knocked out. They’re less realistic, less effective as research tools.
But here’s a clue: iPS cells from ALS patients show a mutant protein in the motor neuron, but it’s not at a sky-high level, unlike what we see in the animal model. In animals, when we have a load of misshapen proteins, the cell’s response is not an authentic replica of what happens in the human body. It’s misleading. That discovery led to the next step, which was to build a drug screening platform to test drugs. Once you think you know the mechanism, it becomes much easier to evaluate thousands of compounds very quickly, and then you are doing it in human cells. When you look at patient cells directly, you see the real thing.
Where do you see the field in another 5–10 years?
There should be something in the clinic. I’d bet on a few conditions with local pathology or a defined cell type, such as Parkinson’s, spinal cord injury, and possibly Huntington’s disease or even stroke. For some disorders, cell therapy could remain quite difficult. Alzheimer’s, for example, is a very diffuse wiring problem.
But I also see that the basic science is setting us up for more victories further down the road. Ask anyone in neuroscience: The need has never been greater.
Tags: animal research, brain, diseases, health, stem cells




