Sequences capture the code of the common cold
In an effort to confront our most familiar malady, scientists have deciphered the instruction manual for the common cold.
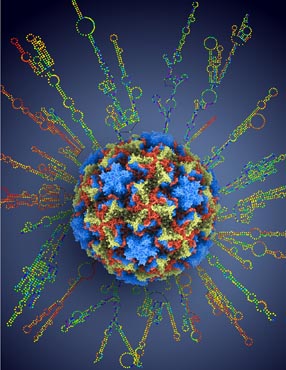
A multi-institutional team of researchers has deciphered the complete genetic sequences of all of the world’s 99 known strains of human rhinovirus, the viruses responsible for the common cold. The sequences provide a detailed blueprint for the virus, showing how new strains develop and revealing pressure points that may lead to new antiviral drugs. Here, a rhinovirus particle at atomic resolution is superimposed over a representation of the RNA molecule that encodes the virus genome.
Image courtesy Ann Palmenberg, Jean-Yves Sgro and H. Adam Steinberg
Writing this week (Feb. 12) in the journal Science, a multi-institutional team of researchers reports the sequences for all of the 99 known strains of cold virus, nature’s most ubiquitous human pathogen. The feat exposes, in precise detail, all of the molecular features of the many variations of the virus responsible for the common cold, the inescapable ailment that makes us all sneeze, cough and sniffle with regularity.
Conducted by teams at the University of Maryland School of Medicine, UW–Madison and the J. Craig Venter Institute, the work to sequence and analyze the cold virus genomes lays a foundation for understanding the virus, its evolution and three-dimensional structure and, most importantly, for exposing vulnerabilities that could lead to the first effective cold remedies.
“We’ve had bits and pieces of these things for a long time,” says Ann Palmenberg, of UW–Madison’s Institute for Molecular Virology and the lead author of the new study. “Now, we have the full genome sequences and we can put them into evolutionary perspective.”
As its name implies, the common cold is an inescapable, highly contagious pathogen. Humans are constantly exposed to cold viruses, and each year adults may endure two to four infections, while schoolchildren can catch as many as 10 colds.
“We know a lot about the common cold virus,” Palmenberg explains, “but we didn’t know how their genomes encoded all that information. Now we do, and all kinds of new things are falling out.”
The genetic sequence of an organism is, in essence, a blueprint that carries all the necessary information for life. It reveals at the most basic level how an organism is constructed and can help scientists look back in time, assemble a family tree and see how a plant, animal or microbe came to be. With pathogens such as viruses, it can also be used to help predict the potential virulence of new emerging agents of disease.
A sequenced genome can also show an organism’s vulnerabilities. In the case of the cold virus, for example, the sequenced genomes are showing which receptors on cells the viruses bind to, information that can be used to design drugs that could potentially help prevent or mediate infection as viruses require access to host cells to do their dirty work and make new viruses.
“This gives us the molecular basis for drug activity,” says Palmenberg. “We can predict which drugs can take them out.”
Stephen B. Liggett, the new study’s senior author and a professor of medicine and physiology at the University of Maryland School of Medicine, notes that the relative paucity of information about the genetic composition of the many strains of cold virus has slowed the development of effective drugs to prevent infection, medicine that can be critically important for some populations.
“We generally think of colds as a nuisance, but they can be debilitating in the very young and in older individuals, and can trigger asthma attacks at any age,” says Liggett, a pulmonologist and molecular geneticist. The new sequences, he says, may help science understand the etiology of asthma as recent studies suggest rhinovirus infection in children can reprogram the immune system to develop asthma by adolescence.
The newly sequenced viruses also show, says Palmenberg, why it is unlikely we will ever have an effective, all-purpose cold vaccine: The existing reservoir of viruses worldwide is huge and, according to the new study, they have a tendency to swap genetic sequences when cells are infected by more than one virus, a phenomenon that can lead to new virus strains and clinical manifestations.
“Having sequenced the complete genomes of these things we now know you can be infected by more than one virus at a time and that they can recombine (their genes),” Palmenberg explains. “That’s why we’ll never have a vaccine for the common cold. Nature is very efficient at putting different kinds of paint on the viruses.”
The ability of different cold virus strains to swap genes and make entirely new strains was thought to be impossible, notes Claire M. Fraser-Liggett, a co-author of the new study and director of the Institute for Genome Sciences and professor of medicine and microbiology at the University of Maryland School of Medicine. “There is the possibility that this could lead to the emergence of a new rhinovirus strain with fairly dramatic properties,” says Fraser-Liggett.
However, with cold virus sequences in hand, as well as some idea of how they exchange genetic information, it may be possible to predict the pathogenic potential of a virus and devise antiviral agents to thwart infection.
The sequenced cold viruses, which were collected from human noses worldwide, fall primarily into two broad species categories or serotypes of human rhinovirus, types A and B. The new work is timely as it presents a framework for understanding yet another newly described species of rhinovirus known as C, whose strains are less common, but far more virulent, capable of infecting cells deep in the lungs.
The new study was funded by the University of Maryland School of Medicine and the National Institutes of Health.