Rare neurological disease shines light on health of essential nerve cells
Ian Duncan is a Scotsman with the iron discipline and stamina of a competitive marathoner, triathlete and cross-country skier. As a neuroscientist at the School of Veterinary Medicine at the University of Wisconsin–Madison, he’s applied his tenacity to a rare genetic disorder.
Known as Pelizaeus Merzbacher disease or PMD, it’s a devastating neurological condition that, in its most severe form, kills infants weeks after birth.

Ian Duncan
Thirty years ago, Duncan noticed a genetic mutation in dogs that was practically identical to the disease in humans. Now, in the online edition of the journal Neurobiology of Disease, Duncan has laid out the results of his marathon pursuit of PMD.
His work underscores the benefits of patience and persistence in the discovery of unexpected results that may point toward new therapies.
Like multiple sclerosis, PMD is a disease that interferes with myelin, the insulation needed for normal communication in the nervous system. The genetic mutation that blocks the formation of myelin is found on the X chromosome, so the disease occurs only in males.
Studies of the dogs are crucial, Duncan says, since PMD is so rare in humans — occurring in one male birth in roughly 200,000.
Duncan says his studies have yielded discoveries that could help fight the grievous illness.
First, Duncan determined that in dogs, myelin in the spinal cord is gradually generated after birth, proving that the cells that make myelin survive and work in some regions. “The brain is acting differently than the spinal cord,” Duncan says. “It’s striking, on day one there is no myelin. But after some months the spinal cord develops myelin and by two years it is almost normal, but the brain is not, and the lack of myelin is the cause of death.”
In these dogs, and presumably in human PMD, there are abnormally few myelin-forming cells, or oligodendrocytes, in the brain and spinal cord. “For reasons we don’t know, the spinal cord repairs because the number of those cells increases with time, but this doesn’t happen in the brain.”
Second, nerve fibers called axons totally lacking myelin have survived for more than two years in the dogs, flouting the accepted wisdom that axons cannot survive without myelin. (Axons are fibers that connect nerve cells to each other, and to muscles.) If lack of myelin is not a death sentence for the axon, the clinical picture for many myelin-related diseases is much brighter, Duncan says.
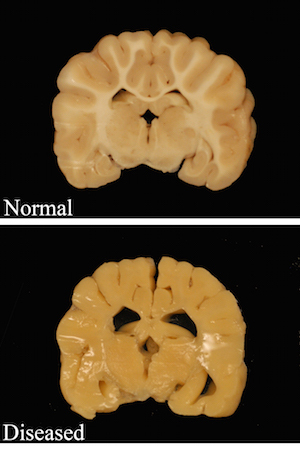
Top: White areas in the brain of a healthy dog show axons with normal myelin sheathing. Bottom: The brain of a dog that models the Pelizaeus Merzbacher disease shows a total absence of myelin insulation.
Photo: Josh Mayer
Third, the recognition that myelin-making cells are still active is shifting Duncan’s focus toward drugs that can increase their number and activity.
“There is hope that several drugs already on the market could stimulate the production of more myelin,” he says. “That could help PMD patients — both human and canine — and potentially MS patients as well.”
That concept is already being tested in MS patients in a phase 1 trial at the University of California, San Francisco.
Unlike MS, PMD is a genetic disorder, suggesting the need for gene therapy. However, Duncan suspects that simply increasing the number of myelin-producing cells early in PMD might cause myelin formation in the brain as well as the spinal cord, eliminating the need for gene therapy.
Finally, Duncan says, his studies make clear that oligodendrocytes — cells that create a protective sheath around myelin — develop differently in the spinal cord than in the brain.
“It’s a big surprise,” he says. “Neurologists have focused on the brain, which is bigger and easier to image.”
Duncan says the dog model of PMD is “strikingly similar to the human disease which we are unable to study in any detail. This is a naturally occurring disease, and our study of it will generate treatments for dogs and for humans alike.”




