In a first, researchers image adaptive immune systems at work in fish
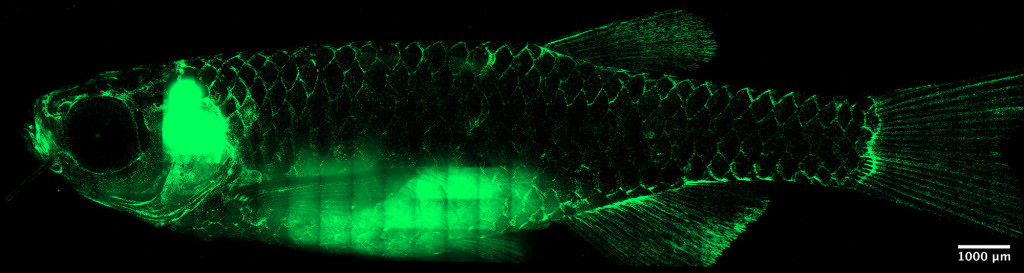
Under a microscope, a 10-week-old zebrafish’s T cells (green) congregate along scale edges, forming a networked immune system across the animal’s body. For the first time, researchers at the University of Wisconsin–Madison have visualized the adaptive immune system of a non-mammal species in stunning detail. Tanner Robertson / University of Wisconsin–Madison
A new study from researchers at the University of Wisconsin–Madison offers a first-of-its-kind visual of a non-mammal species’ adaptive immune system in action. The advance holds potential implications for a range of scientific aims, from improving wildlife vaccines to better understanding fundamental disease processes and possibly the evolution of adaptive immunity itself.
The study, recently published in the Proceedings of the National Academy of Sciences, tracks the movement of immune cells through zebrafish in extraordinary visual detail, revealing the cells’ systematic circulation around the creatures’ bodies — a phenomenon that had never before been documented.
Like many scientific discoveries, the researchers did not originally set out to make it.
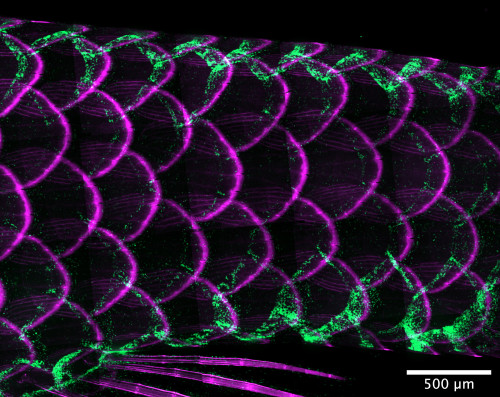
At the base of an adult zebrafish’s tail, called the caudal peduncle, the animal’s tessellated lymphoid network glows green against magenta-tinged scales. Tanner Robertson / University of Wisconsin–Madison
“It was very much unintentional,” says Tanner Robertson, a postdoctoral researcher in the Department of Medical Microbiology and Immunology who led the work. Robertson is an immunologist who previously studied human diseases using mouse models. Mice are a useful model in part because, as mammals, they share humans’ network of lymph nodes. The bean-shaped organs play a key role in the adaptive immune systems of mammals. That’s not the case for many other animals, including zebrafish.
“One of the things that confused me about zebrafish, coming from a mouse model,” says Robertson. “They don’t have lymph nodes, so how do their adaptive immune systems work?”
Nearly all vertebrates have adaptive immune systems made up of specialized cells and anatomical networks that ward off pathogens. In humans and other mammals, lymph nodes and lymphatic vessels make up the physical infrastructure by which pathogen-fighting T cells and other immune cells traverse the body and hunt down infectious agents.
On the other hand, while birds, reptiles, amphibians and jawed fish also have adaptive immune systems, they lack lymph nodes for collecting and moving immune cells throughout their bodies. To date, the structure of these infection-fighting systems and how they work in non-mammals has remained relatively opaque.
“It’s hard to understand how their adaptive immune systems could work at all in the absence of lymph nodes,” says Robertson, so he set out to try to do so.
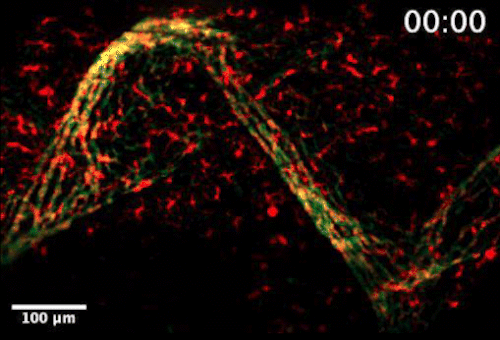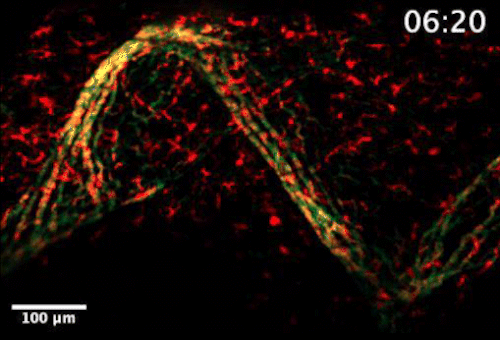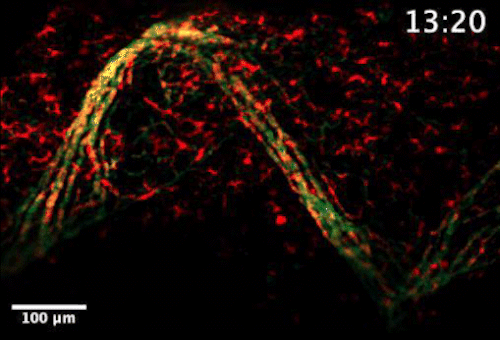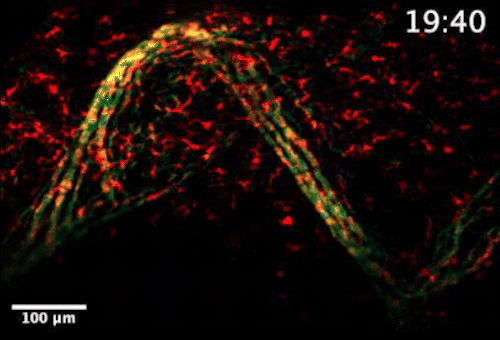
Live video microscopy shows coordinated and collective T cell movement through a zebrafish’s tessellated lymphoid network. Tanner Robertson / University of Wisconsin–Madison
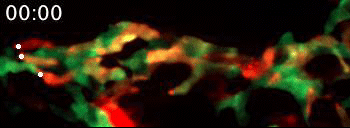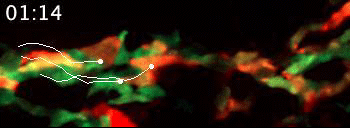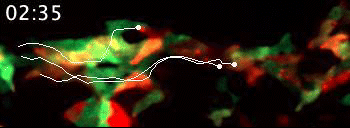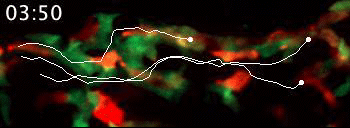
Live video microscopy follows the directional movement of three highlighted T cells within a small portion of the tessellated lymphoid network in a zebrafish. Tanner Robertson / University of Wisconsin–Madison
Using a sophisticated imaging setup and zebrafish that had been genetically altered to remain transparent through adulthood, Robertson and his colleagues were able to track immune cells expressing fluorescent proteins as they traveled through the fish.
What they found was a strikingly organized network of immune cells. In particular, they documented T cells traveling around zebrafish bodies via pockets that form between the fishes’ scales. Within these pockets, the fluorescent cells appeared as a regularly repeating diamond-shaped pattern that mirrors the shape of the scales themselves.
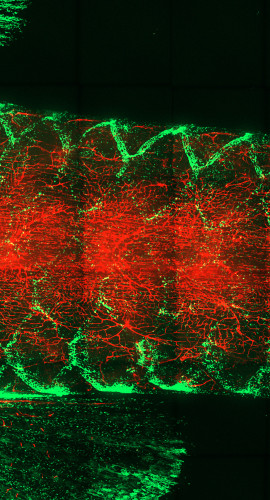
At the base of an adult zebrafish’s tail, the animal’s T cells glow green against the red of its blood vessels. Tanner Robertson / University of Wisconsin–Madison
“We discovered that these cells can move in this process called collective migration, which is a very efficient way for cells to move quickly,” says Anna Huttenlocher, a UW–Madison professor of medical microbiology and immunology and pediatrics who advised Robertson on the study.
Previous studies have suggested that T cells in other animals including mammals might migrate around the body in a similar fashion, but researchers have never directly observed the phenomenon.
“To our knowledge, a network that organizes T cells into a repeating pattern has never been observed in any organism,” says Huttenlocher.
The researchers found that this orderly T cell traffic functions similarly to mammalian lymph nodes. When the fish are infected, the behavior of the T cells transitions so that instead of circulating continuously through the zebrafishes’ bodies, their movements become more random as they search for antigens.
While the new study sheds some light on how adaptive immune systems function in zebrafish, it also presents researchers with a number of exciting questions to pursue.
“This is one of those projects where I think we have generated more questions than we’ve answered,” says Robertson. “We don’t know if the network that we described here is something that exists in other animals. It’s getting at an important academic question about the way that the immune system evolved.”
Tags: animal research, evolution, microbiology, wildlife